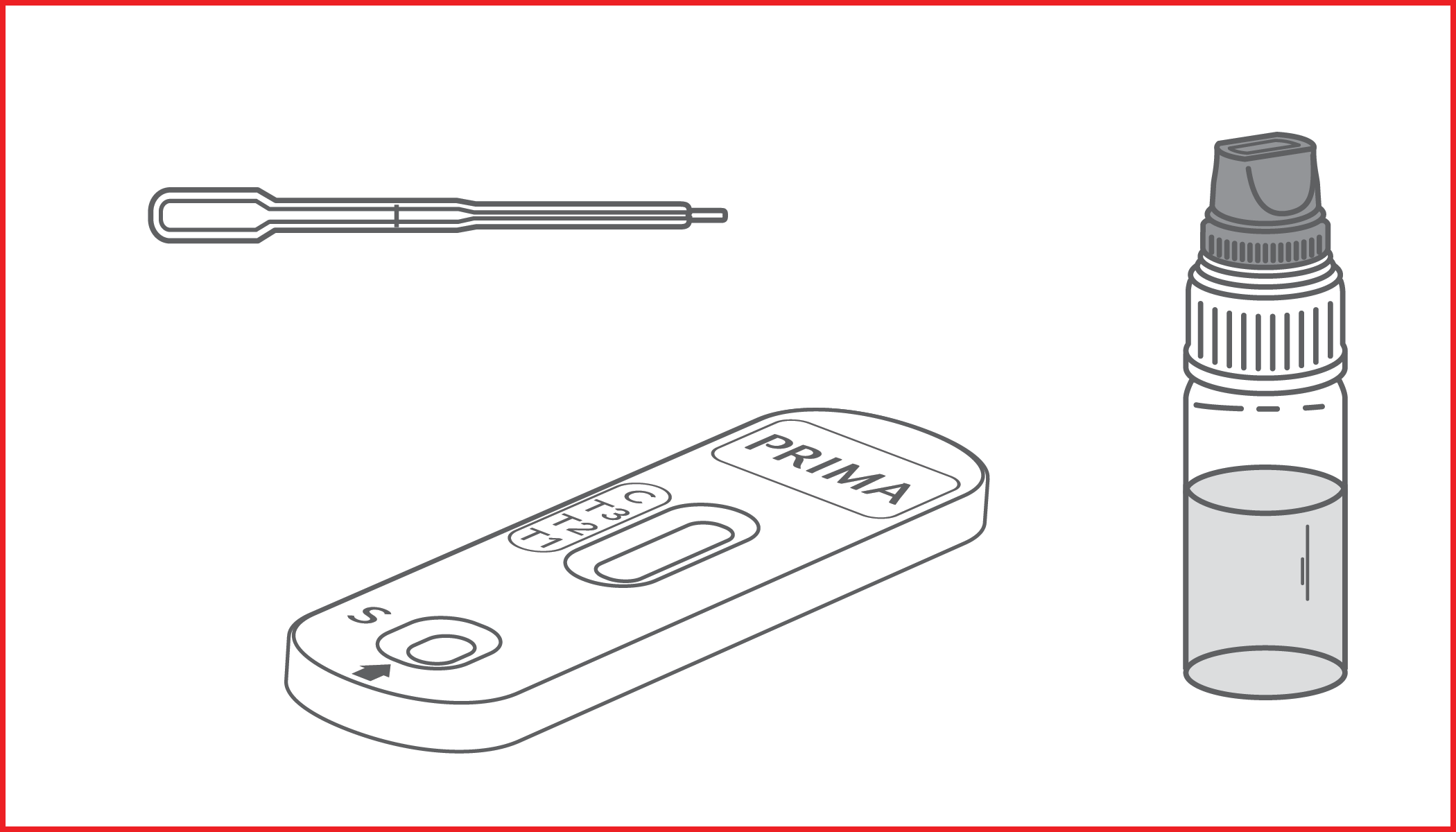
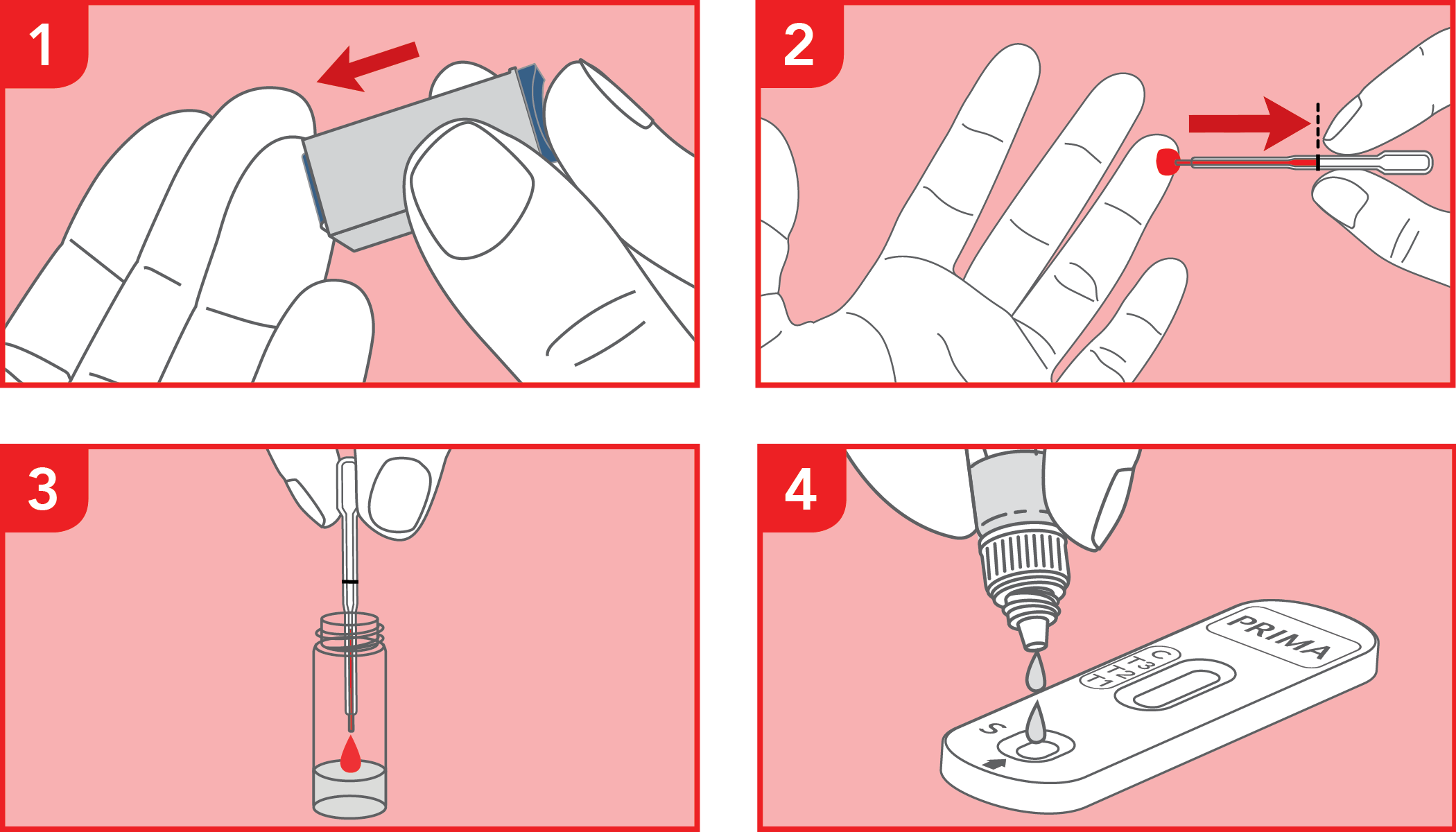
Rapid immunochromatographic test for the semi-quantitative detection of C-Reactive Protein (CRP) in whole blood or serum samples. IVD for professional use only.

C-Reactive Protein (CRP) is an acute phase protein produced mainly by the liver, the concentration of which increases as a consequence of injuries, infections and inflammations.
read moreAlthough it is not a specific marker for a particular pathology, CRP is used as a generic diagnostic indicator of infections and inflammations, as well as to monitor patients’ responses to therapies and postoperative course.
Levels of this protein in blood are high during bacterial infections, while they are restrained during viral infections. For this reason C-Reactive Protein assay can be a useful tool in defining the cause of an inflammation.
Easy to use
Results in 5 minutes
Rapid support to healthcare personnel
C-REACTIVE PROTEIN RAPID TEST is useful in assisting the diagnosis and therapeutic monitoring, as it helps to determine the origin of a possible infection.
read moreLevels of C-Reactive Protein in blood reach their maximum concentration between 24 and 48 hours after the onset of the first symptoms of infection/inflammation (e.g., fever, headache, fatigue), then its levels decrease as the infection subsides.
C-REACTIVE PROTEIN RAPID TEST is a rapid immunochromatographic device for the semi-quantitative detection of C-Reactive Protein in whole blood samples.
| Cut-off | 10 mg/L |
| Specificity | 96.00 % |
| Sensitivity | 98.70% |
| Accuracy | 97.60% |



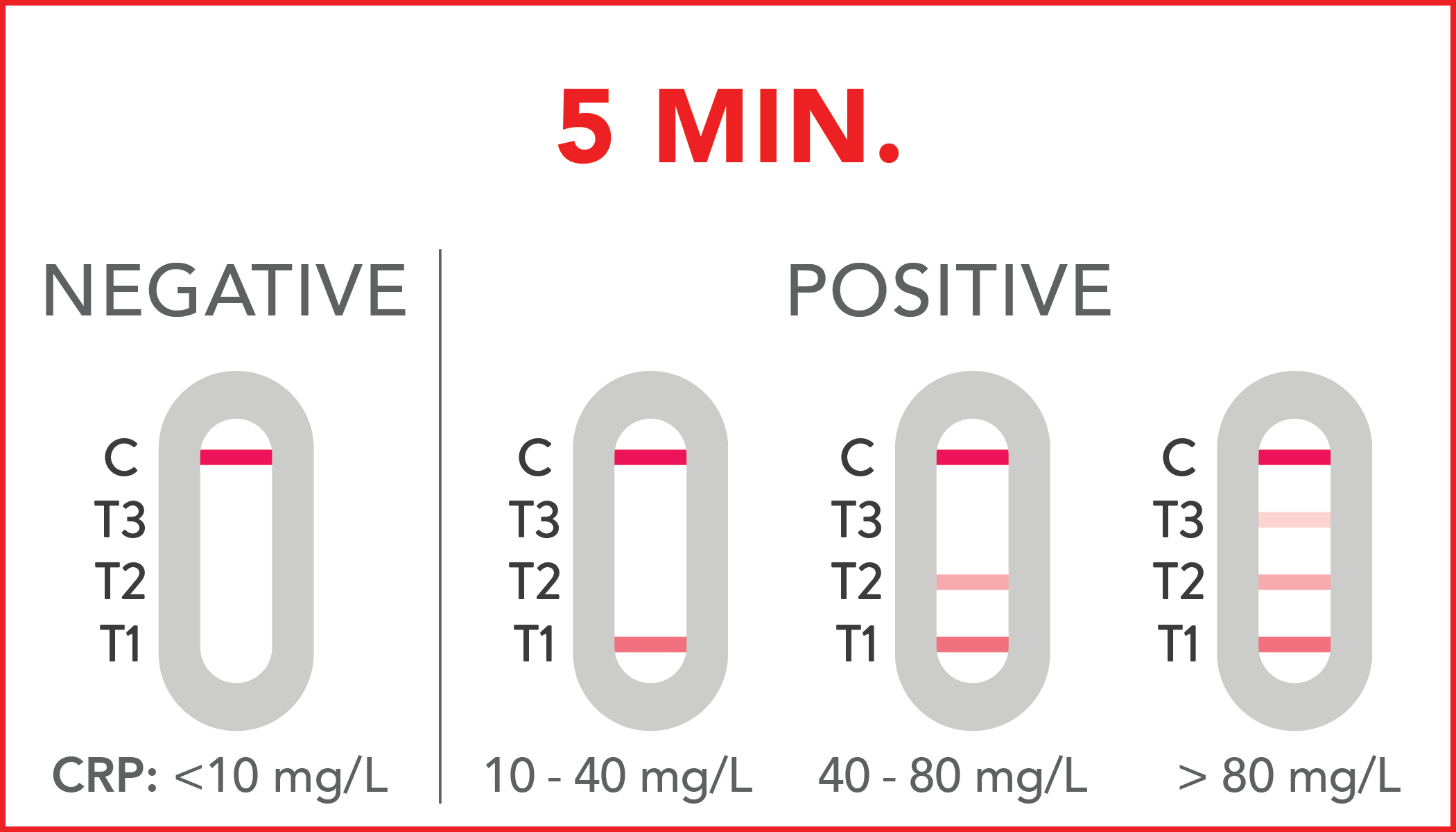
Negative (<10 mg/L): CRP concentration is less than 10 mg/L, suggesting the absence of an infection/inflammation.
Positive (10-40 mg/L): these values are generally associated with an infection of viral origin or the onset of a bacterial infection.
Positive (40-80 mg/L): this result may be associated with a viral or bacterial infection or physical trauma.
Positive (> 80 mg/L): this result may be associated with bacterial inflammation or severe inflammation.
1. C-reactive protein concentrations as a marker of inflammation or infection for interpreting biomarkers of micronutrient status. Vitamin and Mineral Nutrition Information System. Geneva: World Health Organization; 2014 (WHO/NMH/NHD/EPG/14.7).
2. Calarco et al. 2023. “Analytical Performance of 17 Commercially Available Point-of-Care Tests for CRP to Support Patient Management at Lower Levels of the Health System." PLOS ONE 18 (1): e0267516.
3. Dittrich et al. (2016) “Target Product Profile for a Diagnostic Assay to Differentiate between Bacterial and Non-Bacterial Infections and Reduce Antimicrobial Overuse in Resource- Limited Settings: An Expert Consensus”. PLoS ONE 11(8): e0161721. doi:10.1371/journal.pone.0161721.
4. Martínez-González et al. 2020. “Point-of-Care C-Reactive Protein Testing to Reduce Antibiotic Prescribing for Respiratory Tract Infections in Primary Care: Systematic Review and Meta-Analysis of Randomised Controlled Trials.” Antibiotics (Basel, Switzerland) 9 (9): 610.
The test has been carried out correctly when the instructions for use are followed. It includes the reading time and the interpretation of the results shown at the "RESULTS INTERPRETATION" section of the instructions for use.
A colored line will appear at the control region (C) on the test device, showing that the test performed correctly. The absence of the colored line suggests to repeat the test with a new device and a new sample.
The color and intensity of the lines are important for result interpretation: refer to the "RESULTS INTERPRETATION" section of the instructions for use to correctly evaluate the intensity of the test lines (T1, T2 & T3).
Check product availability with the local representative in your country