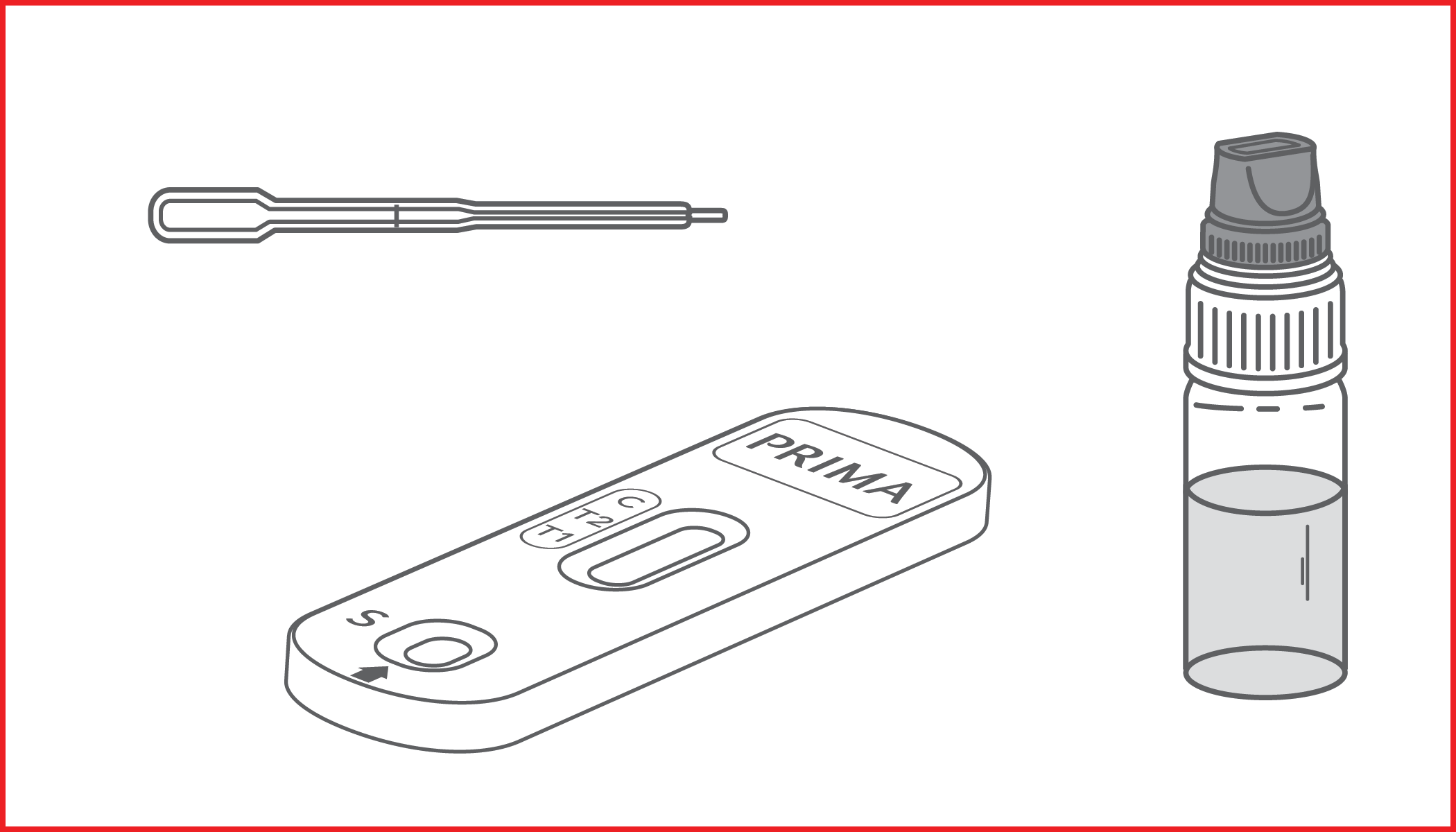
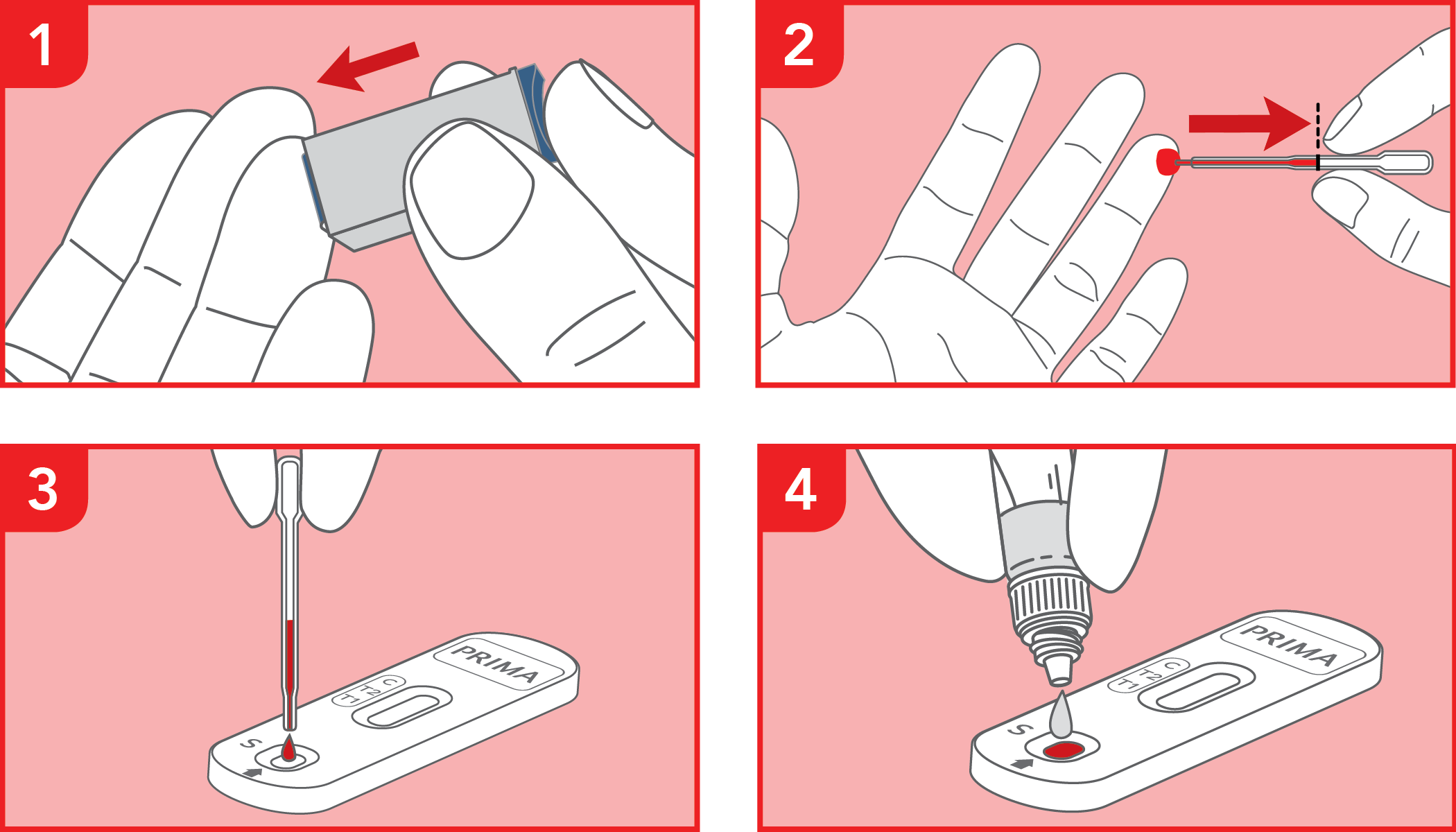
Rapid test for the qualitative detection of IgG anti-gliadin deamidated peptide (DGP) and IgA anti-tissue transglutaminase (tTG) antibodies in whole blood, serum or plasma samples for screening of celiac disease. IVD for professional use only.

Celiac disease is a chronic autoimmune disorder that involves the inflammation of the small intestine due to gliadin intake: the protein portion of gluten found in various cereals (oats, wheat, barley, kamut, spelled, rye, spelled and triticale).
read moreGliadin is a water-soluble peptide that acts as “glue”, helping to maintain the shape of food, and able to pass through the intestinal epithelium. The distinguishing characteristic of celiac disease compared to other small intestinal diseases is the presence of specific antibodies. The determination of circulating levels of immunoglobulin A (IgA) against tissue transglutaminase (tTG) and immunoglobulin G (IgG) against gliadin deamidated pepdide (DGP), currently plays a key role in screening for Celiac disease, as reported in several international guidelines.
Easy to use
Results in 10 minutes
Rapid support to healthcare personnel
CELIAC RAPID TEST is useful for professionals who wants to detect possible celiac disease, which must then be confirmed by further examinations.
read moreIn particular, it is addressed to all those who have symptoms related to celiac disease, such as abdominal pain and swelling, chronic diarrhea, constipation, vomiting, weight loss and irritability, or for those who are genetically familiar with the disease. Even in the absence of the symptoms listed above, it is useful in case of iron, vitamin D, vitamin B or folate deficiency, which could indicate an incorrect intake of essential nutrients.
CELIAC RAPID TEST is a lateral flow immunochromatographic device, for the detection of IgG anti-gliadin deamidated peptide (DGP) and IgA anti-tissue transglutaminase (tTG) antibodies in whole blood or serum/plasma.
| Specificity T1 (IgG anti-DGP) | 90.10% |
| Sensitivity T1 | 84.30% |
| Accuracy T1 | 86.94% |
| Specificity T2 (IgA anti-tTG) | 98.02% |
| Sensitivity T2 | 98.31% |
| Accuracy T2 | 98.17% |



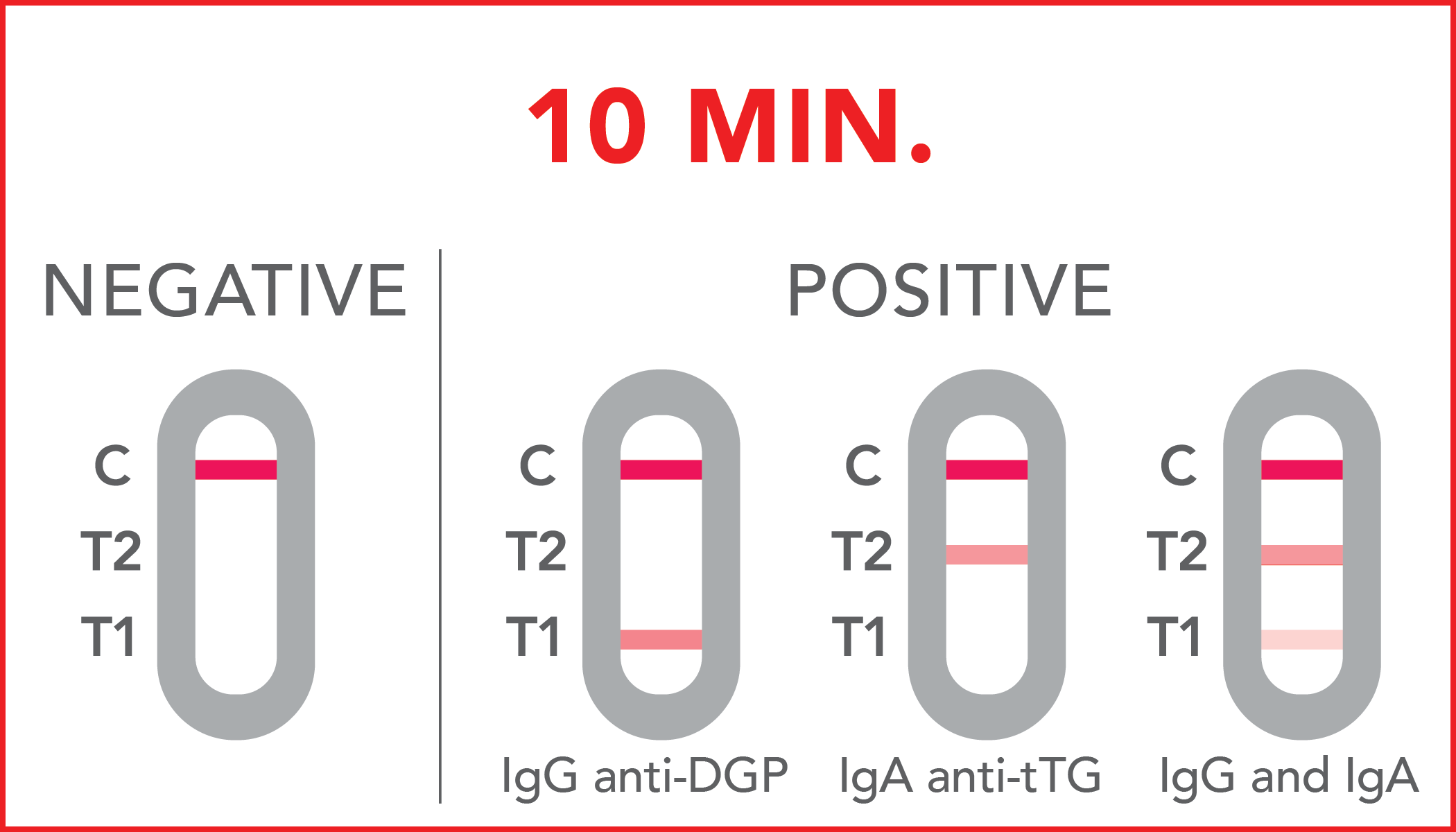
Negative: antibodies are either not present or are present in very low concentrations, undetectable by the rapid test.
IgG anti-DGP Positive: anti-DGP antibodies have been detected in the sample, thus a possible celiac disease, especially if the results are accompanied by the typical symptoms of the disease.
IgA anti-tTG Positive: anti-tTG antibodies have been detected in the sample, thus a possible celiac disease, especially if the results are accompanied by the typical symptoms of the disease.
IgG and IgA Positive: anti-DGPand anti-tTG antibodies have been detected in the sample, thus a possible celiac disease, especially if the results are accompanied by the typical symptoms of the disease.
1. King, et al. 2020. Incidence of Celiac Disease Is Increasing Over Time: A Systematic Review and Meta-Analysis. The American Journal of Gastroenterology 115 (4): 507–25.
2. Lindfors, et al. Celiac disease. Nat Rev Dis Primers 5, 3 (2019).
3. Husby, Steffen, Sibylle Koletzko, Ilma Korponay-Szabó, Kalle Kurppa, Maria Luisa Mearin, Carmen Ribes-Koninckx, Raanan Shamir, et al. 2020. “European Society Paediatric Gastroenterology, Hepatology and Nutrition Guidelines for Diagnosing Celiac Disease 2020”. Journal of Pediatric Gastroenterology and Nutrition 70 (1): 141–56.
4. Al-Toma, et al. European Society for the Study of Celiac Disease (ESsCD) guideline for Celiac disease and other gluten-related disorders. United European Gastroenterol J. 2019 Jun;7(5):583-613.
The test has been carried out correctly when the instructions for use are followed. It includes the reading time and the interpretation of the results shown at the "RESULTS INTERPRETATION" section of the instructions for use.
A colored line will appear at the control region (C) on the test device, showing that the test performed correctly. The absence of the colored line suggests to repeat the test with a new device and a new sample.
The color and intensity of the lines are important for result interpretation: refer to the "RESULTS INTERPRETATION" section of the instructions for use to correctly evaluate the intensity of the test lines (T1, T2 & T3).
Check product availability with the local representative in your country