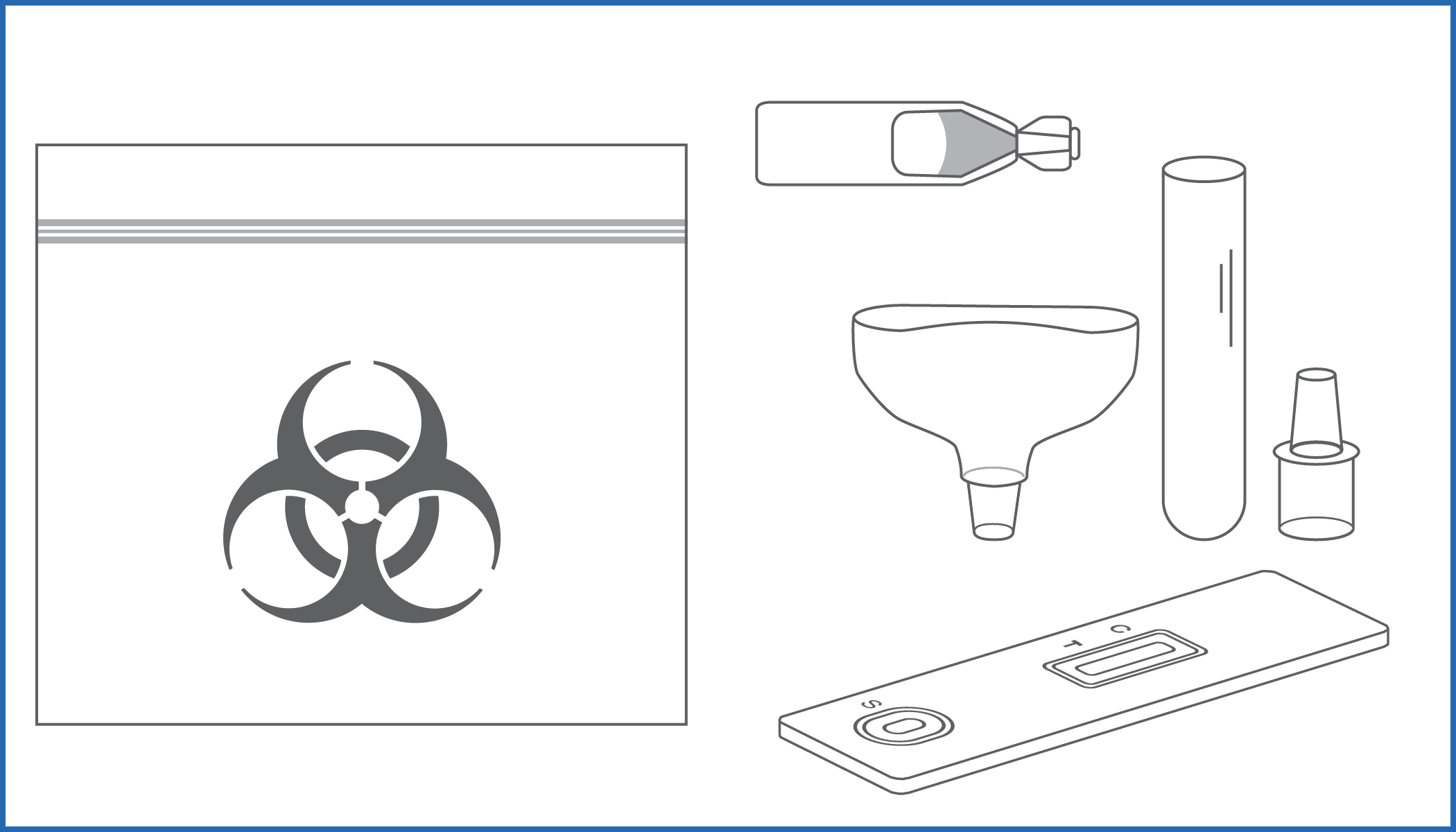
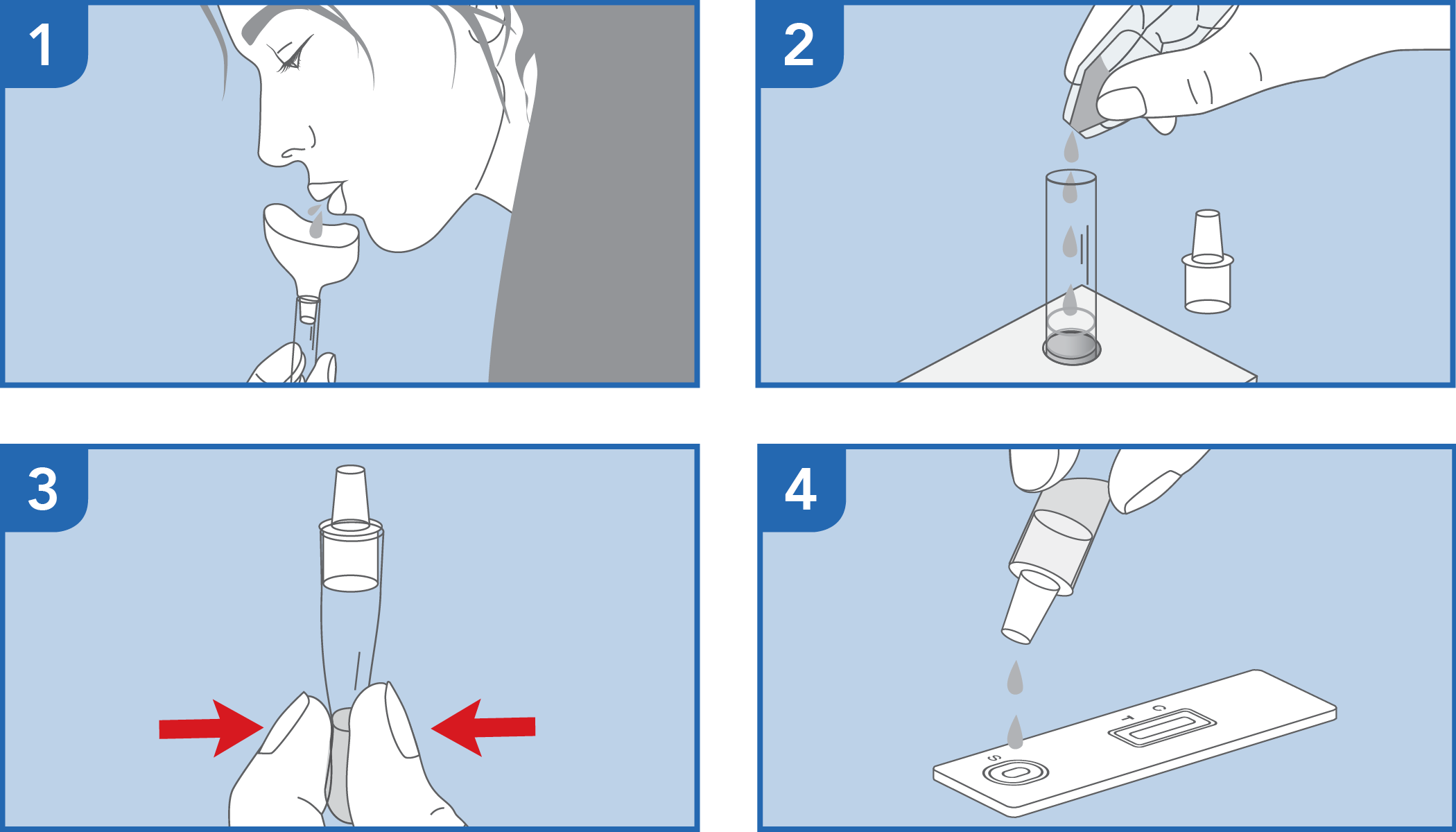
Rapid self-test for the qualitative detection of SARS-CoV-2 antigens present in human oral fluid specimens

Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) has been recognized as the virus responsible for the COVID-19 pandemic.
read moreAs with other respiratory diseases, SARS-CoV-2 can cause an asymptomatic infection, causing mild symptoms such as cold, sore throat, cough and fever, loss of taste and/or sense of smell, or more severe symptoms such as pneumonia and respiratory distress with a fatal outcome. The incubation period of SARS-CoV-2 varies from 1 to 14 days, with an average of 5 to 6 days.
COVID-19 SALIVA SELF-TEST is dedicated for anyone who wants to verify a possible SARS-CoV-2 ongoing infection.
read moreAntigenic tests for COVID-19 allow to carry out a qualitative detection of SARS-CoV-2 in the salivary sample and are configured as rapid diagnostic devices to be carried out for home use. It is recommended to use COVID-19 SALIVA SELF-TEST during the time frame relating to possible exposure to the virus.
COVID-19 SALIVA SELF-TEST is an immunochromatographic assay for the qualitative detection of Nucleocapsid antigens in oral fluid specimens.
| Specificity | 99.3% |
| Sensitivity | 90.1% |
| Accuracy | 97% |



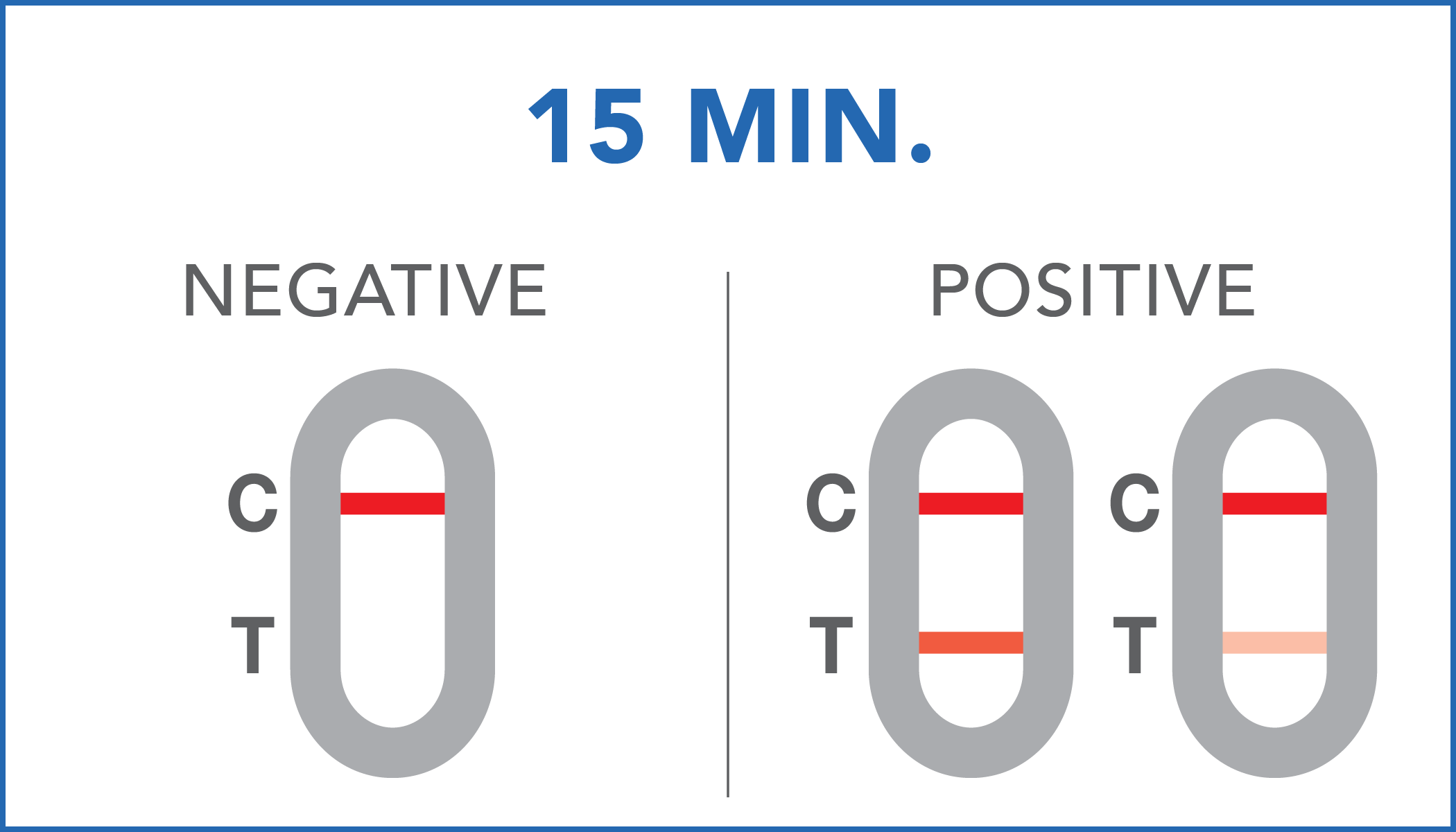
| Negative | SARS-CoV-2 antigens levels are not present in the sample or they are present in very low concentrations, which are not detectable by the device. |
| Positive | SARS-CoV-2 antigens have been detected by the diagnostic system. It is suggested to contact a specialist or the local health department for further information. |
1. BACKINGER, C.L. and KINGSLEY, P.A., Recommendations for Developing User Instruction Manuals for Medical Devices Used in 9 /
57 Home Health Care, Rockville, MD, U.S. Food and Drug Administration, Center for Devices and Radiological Health, HHS Pub. FDA 93-4258.
2. WHO, Advice on the use of point-of-care immunodiagnostic tests for COVID-19 (https://www.who.int/newsroom/commentaries/detail/advice-on-the-use-of-point-of-careimmunodiagnostic-tests-for-covid-19)
3. European Centre for Disease Prevention and Control. Diagnostic testing and screening for SARS-CoV-2. 2020. https://www.ecdc.europa.eu/en/covid-19/latest-evidence/diagnostictesting. Accessed January 2022.
The test has been carried out correctly when the instructions for use are followed. It includes the reading time and the interpretation of the results shown at the "RESULTS INTERPRETATION" section of the instructions for use.
It is advisable to perform the test in the presence of symptoms attributable to COVID-19, or if it is suspected that you have come into contact with positive subjects in the last 5 days.
The color intensity of the control and test bands may vary depending on the concentration of antigens present in the sample. Therefore, any color hue in the test region (T) should be considered as positive.
Check product availability with the local representative in your country