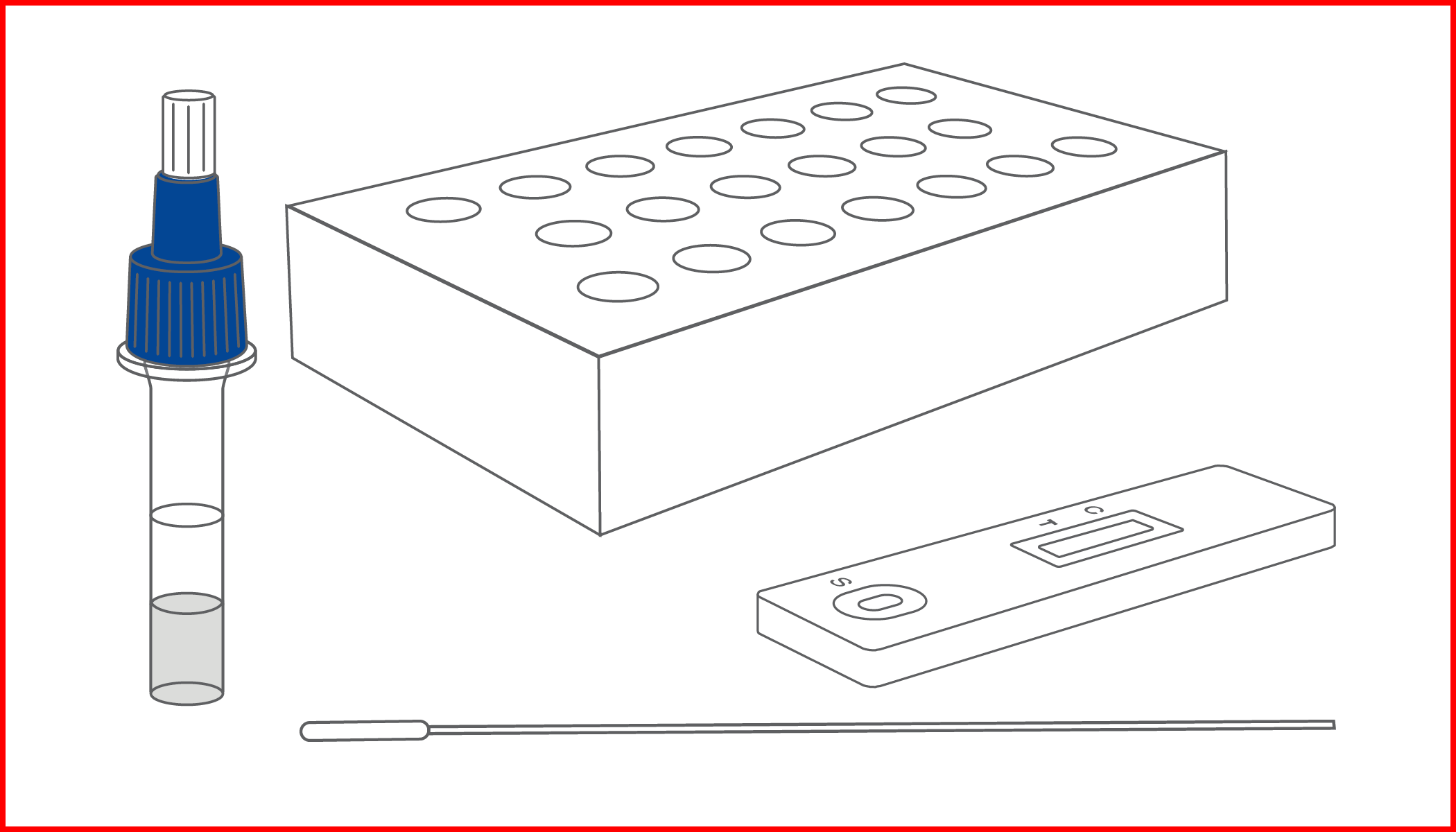
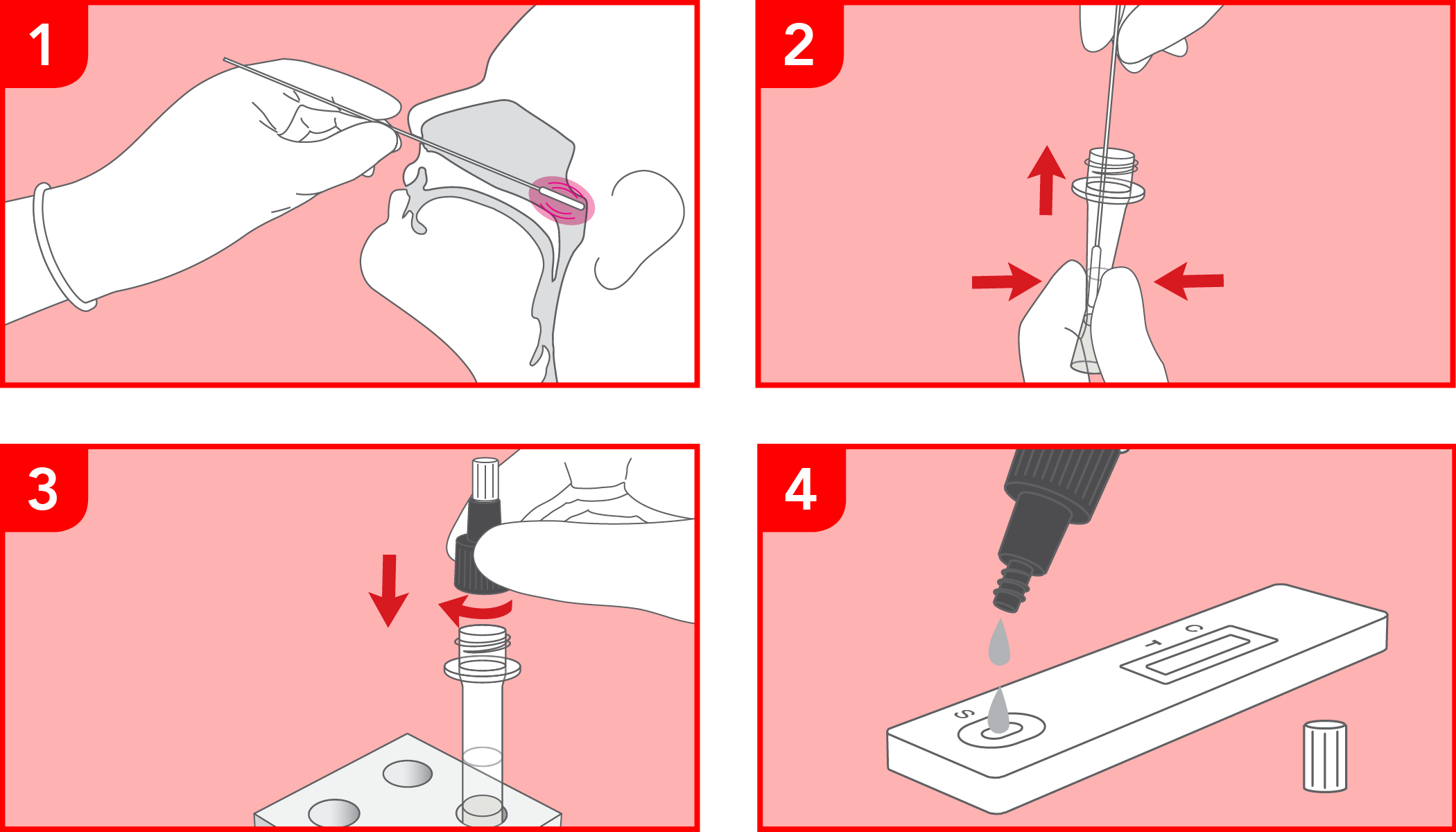
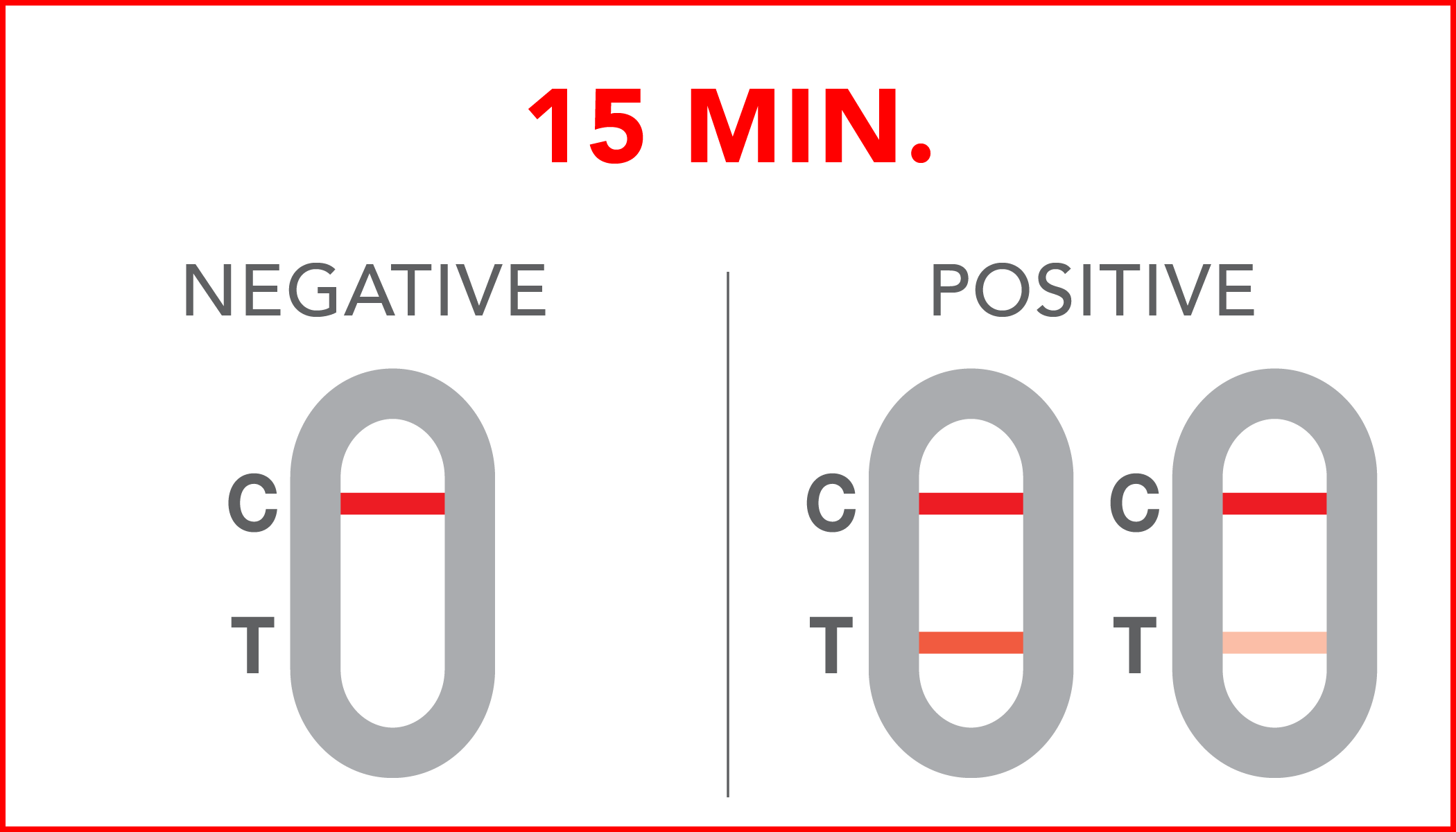
Rapid test for the qualitative detection of SARS-CoV-2 antigens in human nasopharyngeal specimens. For professional in-vitro diagnostic use only.
Listed on EU Common and Swiss Confederation list.

In December 2019 cases of pneumonia of unknown etiology were reported in Wuhan, Hubei Province, China. In January 2020, the Chinese Center for Disease Control and Prevention (CDC) identified Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) as the causative agent of this first outbreak and the related disease was defined as Coronavirus Disease 2019 (COVID-19).
read moreAs well as other respiratory diseases, SARS-CoV-2 can cause an asymptomatic infection, mild symptoms such as cold, sore throat, cough and fever, loss of taste and/or sense of smell, or more severe symptoms such as pneumonia and respiratory distress with a fatal outcome. The incubation period of SARS-CoV-2 varies from 1 to 14 days, with an average of 3 to 7 days.
The CDC, the US Center for Disease Control and Prevention, recommends that samples taken from the upper respiratory tract, such as nasopharyngeal ones, have to be collected and tested in order to identify the presence of genetic material or antigens of the pathogen. In particular, antigenic tests detect the presence of proteins that are structural and functional components of the pathogen itself and are very specific to the target microorganism.
Easy to use
Results in 15 minutes
COVID-19 emergency support
COVID-19 ANTIGEN RAPID TEST is intended for use by healthcare professionals as a first approach in the diagnosis of COVID-19, with the aim of supporting personnel in emergency management with timely responses, in just 15 minutes.
read moreAntigenic tests for COVID-19 allow to carry out a qualitative detection of SARS-CoV-2 in the patient sample and are intended as rapid diagnostic devices to be carried out in the point of care. The incubation period for SARS-CoV-2 virus can range from 1 to 14 days, with an average of 5 to 6 days. It is therefore recommended to use this device during this timeframe starting from possible exposure to the virus.
COVID-19 ANTIGEN RAPID TEST is a rapid lateral flow immunochromatographic device for the detection of SARS-CoV-2 antigens in human nasopharyngeal swab samples. During the test, any SARS-CoV-2 antigens present in the sample react with the anti-SARS-CoV-2 antibody coated nanoparticles present in the test strip. A positive result indicates that the antigen concentration is close to the Limit Of Detection (LOD).
| Test format | rapid immunochromatographic lateral-flow assay |
| Results reading time | 15 minutes |
| Sample | nasopharyngeal swab |
| Antigen | Nucleocapsid |
| Specificity | 99.9% |
| Sensitivity | 93.4% |
| Accuracy | 99.6% |



| Negative | no SARS-CoV-2 antigens have been detected in the sample or their concentration is below the Limit of Detection. |
| Positive | SARS-CoV-2 antigens have been detected in the sample, thus a possible ongoing infection. |
1. European Centre for Disease Prevention and Control, Disease background of COVID-19 (https://www.ecdc.europa.eu/en/2019-ncov-background-disease)
2. WHO Director-General’s opening remarks at the media briefing on COVID-19 - 11 March 2020 (https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020)
3. Centers for Disease Control and Prevention, Interim Guidelines for Collecting, Handling, and Testing Clinical Specimens for COVID-19 (https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specimens.html)
4. European Centre for Disease Prevention and Control. Diagnostic testing and screening for SARS-CoV-2. 2020. https://www.ecdc.europa.eu/en/covid-19/latest-evidence/diagnostic-testing. Accessed January 2022.
5. https://www.who.int/news-room/commentaries/detail/advice-on-the-use-of-point-of-care-immunodiagnostic-tests-for-covid-19
The test has been carried out correctly when the instructions for use are followed. It includes the reading time and the interpretation of the results shown at the "RESULTS INTERPRETATION" section of the instructions for use.
A colored line will appear at the control region (C) on the test device, showing that the test performed correctly. The absence of the colored line suggests to repeat the test with a new device and a new sample.
The color and intensity of the lines do not affect the interpretation of the result. The test has to be considered positive regardless of the color intensity of the test line (T).
Check product availability with the local representative in your country